Abstract
Introduction: Cytomegalovirus infection continues to be a major cause of morbidity and mortality after allogeneic stem cell transplantation (allo-SCT) in spite of recently approved prophylactic and pre-emptive therapies. The use of virus specific T-cells (VST-cells) to treat these infections has shown promising results but their use is hampered by the availability of family and third-party donors as well as timely and logistic issues.
Methods: We conducted a single-arm, open-label phase Ib/II clinical trial (NCT04018261) to evaluate the use of Viro-T-cell, an academic allogeneic VST-cell product against relapsed/refractory (r/r) CMV infections in patients after allo-SCT. r/r CMV infection was defined as plasma CMV reactivation not responding after 2 lines of therapy or organ CMV disease not responding to first line therapy or patients with known genetic mutations conferring resistance to antiviral therapy. Viro-T-cell was generated from peripheral blood lymphocytes obtained after lymphapheresis based in CliniMACS® Cytokine Capture System (IFN-gamma) technology through an approved advanced therapy medicinal product (ATMP). Before starting the clinical trial, an allogeneic VST-cell donor registry was created based on the frequent volunteer blood donors registry in our region. For each patient, the best available and timely donor was identified among family donors and within the donor registry to undergo ATMP manufacturing procedure. The primary end-point of the study was safety. Clinical endpoints were assessed 8 weeks post infusion and patients were followed thereafter.
Results: A total of 263 volunteer donors were enrolled in the VST-cell registry among 2034 frequent blood donors based on HLA genotyping and serological markers. According to our HLA database, these VST-cell donors covered the most frequent of HLA haplotypes in our region. Regarding the clinical trial, twenty-six patients with r/r CMV infection after allo-HCT were screened and 20 were finally included in the trial between September 2019 and August 2021. Of the 20 patients included (90% Caucasian), median age was 54.8 years (IQR 50.2;62.8). Ten patients had received allo-SCT for acute leukemia and most of them (85%) were in complete remission of their base-line disease. Median HCT-CI score was 2 (IQR 1-3). The majority of the patients (85%) received an allo-SCT from unrelated donors and received reduced-intensity conditioning (60%) using peripheral blood as stem cell source (85%). Ten (50%) patients received in vivo or ex vivo T-cell depletion. All patients had detectable viral load in plasma at Viro-T-cell infusion (median 2191 IU/ml [IQR 803.5;6630.5 IU/mL]). Viro-T-cell was administered to all patients included in the trial and was produced using T-cells from the same allo-SCT donor (n=5, 25%) or from a third-party donor (n=15, 75%), including close family members (n=7) or volunteer donors from the registry (n= 8). Median time from inclusion in the trial to infusion of Viro-T-cell was 8.5 days (IQR 6;20) and median number of cells in the product was 0.98x10E4/Kg (IQR 0.4;1.58) with a median viability of 97.5% (IQR 93.3;98.8). Median percentage of CMV reactive T-cells in the product was 61.1% (IQR 36.3-66.4).
Viro-T-cell was generally well tolerated. Globally, 133 adverse events (AE) were observed in 18 patients (90%) and 126 of them were considered not related with the investigational product. Only 11 AEs were considered serious AE, mostly infectious (n=5) and hematologic (n=3) events. Graft-versus-host disease was observed in one patient after Viro-T-cell infusion (Stage 1 skin).
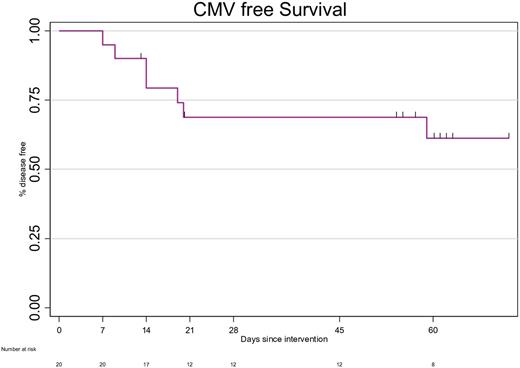
In terms of efficacy, 14 patients responded to Viro-T-cell for a probability of response at week 8 of 61.18% (95%CI 34.50-79.69) (Figure1). None of these patients required further anti-CMV therapy after infusion. Higher absolute leukocyte (HR 1.41 [95% CI 1.04;1.90], P=0.03) and lower terminal CD4+ cell counts (HR 0.14 [95% CI 0.02;1], P=0.05) at infusion were associated with higher probability of response to Viro-T-cell. CMV reactivity based on ELISPOT increased from 65% of patients before infusion to 83% at week 8 after therapy.
Conclusions: Viro-T-cell, an academic viral specific T-cell product is safe and effective in allo-SCT recipients with r/r CMV infection. The use of a third party donor registry allows treating the majority of patients with r/r CMV infections after allo-SCT by using the best available donor for each patient.
Disclosures
Barba:Pfizer: Honoraria; Novartis: Honoraria; Gilead: Honoraria; BMS: Honoraria; Amgen: Honoraria. Mussetti:BMS: Consultancy; GILEAD: Research Funding; TAKEDA: Honoraria; JAZZ PHARMACEUTICALS: Consultancy. Ferra:Janssen, Roche, Gilead, Takeda, Abbvie: Consultancy, Other: Medical meetings funding. Bosch:Roche: Consultancy, Honoraria; Karyospharm: Honoraria; Takeda: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Mundipharma: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Beigene: Consultancy, Honoraria.
OffLabel Disclosure:
Viral specific T-cell product evaluated in a clinical trial
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal